LEQEMBI
Reimbursement Leqembi
1/6/20232 min read
An injectable monoclonal antibody called LEQEMBI® (lecanemab-irmb) has been authorized for the treatment of Alzheimer's disease (AD). For proper reimbursement and adherence to coverage policies, accurate billing and coding are necessary. The main rules are as follows:
Code for HCPCS:
J0174: Lecanemab-irmb injection, 1 mg.
Example of Billing: Since one unit is equivalent to one milligram, bill 700 units for a 700 mg dose of LEQEMBI.
NDC, or the National Drug Code:
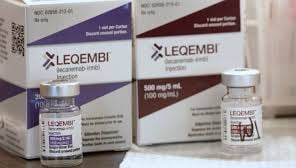
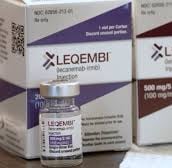
62856-0212-01: One-dose vial of LEQEMBI 200 mg/2 mL (100 mg/mL).
LEQEMBI 500 mg/5 mL (100 mg/mL) single-dose vial, 62856-0215-01.
Alzheimer's disease (AD) can now be treated with LEQEMBI® (lecanemab-irmb), an injectable monoclonal antibody. Accurate billing and coding are required for appropriate reimbursement and compliance with coverage policies. The main rules are as follows:
HCPCS code: J0174: 1 mg injection of lecanemab-irmb.
An illustration of billing Bill 700 units for a 700 mg dose of LEQEMBI because one unit is equal to one milligram.
The National Drug Code, or NDC, is 62856-0212-01: 200 mg/2 mL (100 mg/mL) LEQEMBI one-dose vial.
LEQEMBI single-dose vial, 62856-0215-01, 500 mg/5 mL (100 mg/mL).
Other Things to Think About When Billing: Modifiers Add the proper modification for administering LEQEMBI in a clinical research setting:
Q0: Clinical investigational services rendered inside a clinical research study that has been authorized.
Q1: Standard clinical services rendered in a clinical research study that has been authorized.
Field 19 of the CMS-1500 claim form or Loop 2300 REF02 (REF01=P4) for electronic claims are the appropriate places to report these modifiers.
Invoicing and payment for LEQEMBI treatment. Registry Reporting: In Field 19 of the CMS-1500 claim form or, in the case of electronic claims, in Loop 2300 REF02 (REF01=P4), providers are required to disclose the registry trial number (an eight-digit number, such as "99999999" or a specific NCT number).
Coverage Requirements: If specific requirements are fulfilled, such as patient registration in a registry, LEQEMBI is covered under Medicare's National Coverage Determination (NCD) 200.3 for monoclonal antibodies directed against amyloid for the treatment of AD.
Keep thorough medical records that include the diagnosis, treatment plan, dosage, administration route, and any registry information. This paperwork helps with the processing of claims and shows medical necessity.
For thorough and current details, consult the LEQEMBI Coding Quick Reference Guide offered by
Following these recommendations can help ensure accurate
