FABRAZYME
Reimbursement Fabrazyme
2/6/20081 min read
Adult and juvenile patients with confirmed Fabry disease who are 2 years of age or older can benefit from treatment with the enzyme replacement medication Fabrazyme® (agalsidase beta).
Fabrazyme HCP
For proper reimbursement and compliance, accurate billing and coding are necessary. The main billing and coding rules for Fabrazyme are shown below:
Diagnosis Code: Fabry disease (ICD-10-CM Code: E75.21).
Procedure Codes: J0180 (HCPCS Code): Injection, 1 mg of agalsidase beta. One milligram of Fabrazyme is represented by each billable unit.
CPT® Codes: These codes specify how the medication is administered. 96365: Intravenous infusion for therapy, prophylaxis, or diagnostic; initial, up to 1 hour is one of the frequently used codes.
Every extra hour (List individually in addition to code for principal procedure) is denoted by 96366.
Chemotherapy 96413: Intravenous infusion technique; single or first substance/drug; up to 1 hour.
96415: Every extra hour (list separately, along with the primary procedure code).
The choice between 96365/96366 and 96413/96415 is contingent upon the particular circumstances of the infusion as well as payer guidelines. To find the correct codes, go to the payer policies and the most recent edition of the CPT® handbook.
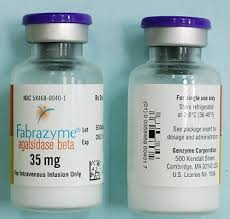
The National Drug Code (NDC) for Fabrazyme 5 mg injection single-dose vial is 58468-0041-01.
58468-0040-01 is a 35 mg injectable vial of Fabrazyme.
For claims processing and payment, it is essential to include the right NDC.
Other Things to Think About When Billing:
Site of Care: Certain payers might have certain rules about where a medication can be administered (e.g., home infusion, doctor's office, or hospital outpatient department). Check with each payer to confirm coverage and authorization needs.
Pre-Authorization: Prior authorization for Fabrazyme therapy is required by many insurance companies. Before starting treatment, make sure that all required approvals have been received.
Documentation: Keep comprehensive medical records that include information on the treatment plan, dose, administration, and the diagnosis of Fabry disease, which is supported by the right tests. This paperwork enables efficient claims processing and substantiates medical necessity.
Consult the manufacturer's Fabrazyme Billing and Coding Guide for complete and current information.
