AMVUTTRA
Reimbursement Amvuttra
6/1/20221 min read

To guarantee correct reimbursement, billing for AMVUTTRA® (vutrisiran) necessitates close attention to coding and documentation. Important things to think about are:
Code for HCPCS:
For AMVUTTRA, use HCPCS code J0225, which is "Injection, vutrisiran, 1 mg." One milligram of vutrisiran is equivalent to one billing unit.
NDC, or the National Drug Code:
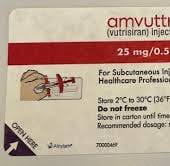
Report AMVUTTRA's 11-digit NDC. The NDC for the single-dose prefilled syringe containing 25 mg/0.5 mL is 71336-1003-01.
Use ICD-10-CM code E85 to diagnose familial transthyretin-mediated amyloidosis with polyneuropathy. Place of Service and Claim Form: The CMS-1500 claim form is used to bill for services rendered in a doctor's office. Make sure you include the appropriate place of service code.
Drug Wastage Modifiers: Beginning with dates of service on or after July 1, 2023, use the JW modifier on Medicare claims to reflect the quantity of drug discarded and the JZ modifier when no medicine is discarded from single-dose vials. Check the policies of individual payers with relation to these modifications.
Documentation and previous Authorization: Acquire previous authorization as needed by payers. Verification of the diagnosis of hereditary transthyretin-mediated amyloidosis should be included in the documentation.
Clinical evaluations that show polyneuropathy.
baseline assessments like the Neuropathy Impairment Score (NIS), the Polyneuropathy Disability (PND) score, or the Familial Amyloid Polyneuropathy (FAP) stage.
Administration Information: A medical practitioner will inject 25 mg of AMVUTTRA subcutaneously once every three months.
Policies Particular to Payers:
For specific billing requirements, which may differ depending on the payer, review their policies. For instance, UnitedHealthcare has particular criteria for RNA-targeted medicines like AMVUTTRA.
Following these rules will make it easier for AMVUTTRA to bill and get reimbursed accurately.
